Electric Charges
Electric Charge
When, one object is rubbed with the another object then number of their electrons are different to number of protons. Thus, one object has more electron than proton and another object has more proton than electron. Object that has more electron gets negative charge and another that has more proton gets positive charge because, electron has negative charge and proton has positive charge. Thus, electric charge is an intrinsic characteristics of the fundamental particles constituting the objects.
Types of Charge
Electric Charges are of two types. American scientist Benjamin Franklin named the two types of charges as positive and negative charges.
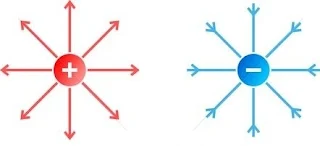
(i) Positive Charge:- If any object lose their electrons then they get positive charge. It is denoted by (+q) sign.
(ii) Negative Charge:- If any object gain electrons from another object then they get negative charge. It is denoted by (-q) sign.
If an object possesses an electric charge (either positive or negative), it is said to be charged or electrified. When an object has no charge, it is said to be neutral. It is clear that although normally the materials are electrically neutral but they contain charges. Of course, their charges are exactly balanced.
Basic Properties of Electric Charge
We have seen that there are two types of charges, namely positive and negative and these charges tend to nullify each other. Charges are said to be point charges if the size of charged bodies are very small as compared to the distance between them.
1. Attraction and repulsion:- Like charges repel each other but unlike charges attract each other.
2. Electric Induction:- When a charged object bring to contact with another uncharged, it gets opposite charge of charged object. It is called charging by induction.
3. Conservation of Charge:- According to this property, "An electric charge neither can be created nor can be destroyed" i.e. total net charge of an isolated system is always conserved. Thus, when a glass rod rubbed with silk cloth, both glass rod and silk cloth acquire opposite charge in same quantity. Thus, total amount of charge remains same before rubbing as well as after rubbing.
4. Additivity of Charge:- As per concept of additivity of charges, if a system contains two or more point charges, the total charge of the system is obtained by simply adding these charges algebraically.
`Q=q_1+q_2+q_3+...+q_n=\sum_{i=1}^nq_i`
5. Effect of velocity on charge:- The velocity does not affect amount of charge of any particle. For example, electron has always 1.6×10-19coulomb charge.
6. Quantization of Charge:- All free charges are integer multiples of a basic unit of charges, which is denoted by e. Thus, charge q on any object must be given by
q = ne
Where n is an integer, e is the charge of an electron. The fact that electric charge is always an integer multiple of e is called the quantization of charge.