Thermal Properties of Matter Class 11 notes Physics Chapter 11
Introduction
In this chapter, we shall examine some of the thermal properties of matter. We will first consider thermal expansion which plays an important role in everyday life and then discuss changes of phase and latent heat. At the end, we will discuss the phenomenon of heat transfer.
When a body is heated, various changes take place. Temperature is a measure of ‘hotness’ of a body. A kettle with boiling water is hotter than a box containing ice. When water boils or freezes, its temperature does not change during these processes even though a great deal of heat is flowing into or out of it.
Temperature and Heat
(i). Temperature
Temperature is a relative measure, or indication of hotness or coldness. A hot cooker is said to have a high temperature, and ice cube to have a lower temperature. An object at a higher temperature is said to be hotter than the one at a lower temperature. The SI unit of temperature is kelvin (K), whereas degree celsius (°C) is a commonly used unit of temperature.
(ii). Heat
When you put a cold spoon into a cup of hot coffee, the spoon warms up and the coffee cools down as they were trying to equalise the temperature. Energy transfer that takes place solely because of a temperature difference is called heat flow or heat transfer and energy transferred in this way is called heat. The SI unit of heat energy transferred is expressed in joule (J).
Measurement of Temperature
A physical property that changes with temperature is called a thermometric property. When a thermometer is put in contact with a hot body, the mercury expands, increasing the length of the mercury column.
(i). Celsius Scale
It defines ice-point temperature as 0°C and the steam point temperature as 100°C. The space between 0°C and 100°C marks is equally divided into 100 intervals.
Recommended Books
- NCERT Textbook For Class 11 Physics Part 1 & 2
- CBSE All In One Physics Class 11 2022-23 Edition
- Oswaal CBSE Chapterwise Question Bank Class 11 Physics Book
- Modern's abc Plus of Physics for Class-11 (Part I & II)
Read also: Thermodynamics Class 11 Physics Notes Chapter 12
(ii). Fahrenheit Scale
It defines the ice-point temperature as 32°F and the steam point temperature as 212°F. The space between 32°F and 212°F is divided into 180 equal intervals.
(iii). Kelvin Scale
Kelvin Scale is a scale of measuring of temperature, the melting point of ice is taken as 273 K and the boiling point of water as 373 K the space between these two points is divided into 100 equal intervals.
(iv). Relation between Different Scales of Temperatures
To convert a temperature from one scale to the other, we must take into account the fact that zero temperatures of the two scales are not the same.
`\frac{C}{100}=\frac{F-32}{180}=\frac{K-273}{100}=\frac{R}{80}`
Note: The normal temperature of the human body measured on the Celsius scale is 37°C which is 98.6°F.
Read also: The p-Block Elements Class 11 Notes Chemistry Chapter 11
Ideal Gas Equation and Absolute Temperature
(i). Ideal Gas Equation
An equation which follows the law of Boyal, law of Charls and llaw of Avogadro is called ideal gas equation.
At constant temperature,
`V\propto\frac{1}{P}` ...( From Boyal's law)
At constant pressure,
`V\propto\T` ...( From charl's law)
At constant T and P,
`V\propto\n` ...( From Avogadro's law)
By combinig all above equation, we get
`V\propto\frac{Tn}{P}`
`V=\frac{nRT}{P}`
`PV=nRT`
where, n = Number of moles of gas
R = Universal gas constant (R = 8.31 J mol–1 K–1)
P = Pressure of gas
V = Volume of gas
Read also: Conceptual Questions for Class 11 Physics Chapter 11 Thermal Properties of Matter
(ii). Absolute Temperature
The absolute minimum temperature is equal to –273.15ºC. This is also known as absolute zero. Absolute zero is the foundation of the kelvin temperature scale or absolute scale temperature.
Thermal Expansion
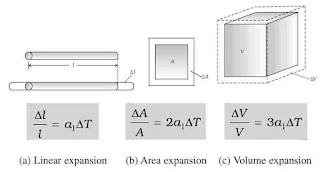
Increase in size of any matter on heating is called thermal expansion. There are three types of thermal expansion.
(i). Linear Expansion
The expansion in length is called linear expansion and the fractional change in length, ΔL/L is given by ΔL/L = αΔT where α is called coefficient of linear expansion.
(ii). Area Expansion
The expansion in area is called area expansion or superficial expansion and the fractional change in area, ΔA/A is given by ΔA/A = βΔT where β is called coefficient of area expansion.
(iii). Volume Expansion
The expansion in volume is called volume expansion and the fractional change in area, ΔV/V is given by ΔV/V = γΔT where γ is called coefficient of volume expansion.
(iv). Relation Between
α : β : γ = 1 : 2 : 3
`\frac{α}{β}=\frac{1}{2}` .....[ β = 2α ]
`\frac{α}{γ}=\frac{1}{3}` .....[ γ = 3α ]
Specific Heat Capacity
If an amount of heat Q, when given to a body of mass m, increases its temperature by an amount ΔT, then
Q = mcΔT
where c is a constant and is called the specific heat capacity or simply specific heat of the material of the body.
If m = 1 kg and ΔT = 1C° then c = Q
Specific heat of the material of a substance is the amount of heat required to raise the temperature of unit mass of the substance through 1C°.
In SI, the unit of c is J/kg K.
Calorimetry
Calorimetry deals with the measurement of heat. The vessel which is largely used in such a measurement is called a calorimeter.
When two bodies at different temperatures are allowed to share heat, they attain a common temperature. If it is assumed that no heat is received from or given to any body outside the system and if there is no chemical action involved in the process of sharing, then
Heat gained = Heat lost
This simple statement based on the law of conservation of energy is called the principle of calorimetry.
Change of State
Depending on temperature and pressure, all matter can exist in a solid, liquid or gaseous state. These states or forms of matter are also called the phases of matter.
The change of state from solid to liquid is called melting and from liquid to solid is called fusion. It is observed that the temperature remains constant until the entire amount of the solid substance melts. That is, both the solid and the liquid states of the substance coexist in thermal equilibrium during the change of states from solid to liquid.
The temperature at which the solid and the liquid states of the substance is in thermal equilibrium with each other is called its melting point. The change of state from liquid to vapour (or gas) is called vaporisation. It is observed that the temperature remains constant until the entire amount of the liquid is converted into vapour.
The temperature at which the liquid and the vapour states of the substance coexist is called its boiling point. The change from solid state to vapour state without passing through the liquid state is called sublimation, and the substance is said to sublime.
Latent Heat
Latent heat is defined as the heat or energy that is absorbed or released during a phase change of a substance. It could either be from a gas to a liquid or liquid to solid and vice versa. Latent heat is related to a heat property called enthalpy. It is denoted by L and its SI unit is J/kg.
`L=\frac{Q}{m}`
There are two types of latent heat.
(i). Latent Heat of melting
It is a amount of heat which is required to change of phase from solid to liquid for unit mass at constant temperature. Ex- Latent heat of melting of ice is 3.33 x 105 J/kg.
(ii). Latent Heat of Vaporization
It is a amount of heat which is required to change of phase from liquid to vapor for unit mass at constant temperature. Ex- Latent heat of vaporization of water is 22.6 x 105 J/kg.
Heat Transfer
There are three mechanisms of heat transfer which name is given as- conduction, convection and radiation. Conduction occurs within a body or between two bodies in contact. Convection depends on motion of mass from one region of space to another. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need for matter to be present in the space between bodies.
(i). Conduction
Conduction is the mechanism of transfer of heat between two adjacent parts of a body because of their temperature difference. Suppose, one end of a metallic rod is put in a flame, the other end of the rod will soon be so hot that you cannot hold it by your bare hands.
Here, heat transfer takes place by conduction from the hot end of the rod through its different parts to the other end. Gases are poor thermal conductors, while liquids have conductivities intermediate between solids and gases.
(ii). Convection
Convection is a mode of heat transfer by actual motion of matter. It is possible only in fluids. Convection can be natural or forced. In natural convection, gravity plays an important part. When a fluid is heated from below, the hot part expands and, therefore, becomes less dense. Because of buoyancy, it rises and the upper colder part replaces it. This again gets heated, rises up and is replaced by the relatively colder part of the fluid. The process goes on.
In forced convection, material is forced to move by a pump or by some other physical means. The common examples of forced convection systems are forced-air heating systems in home.
(iii). Radiation
Radiation is the transfer of heat by electromagnetic waves such as visible light, infrared, and ultraviolet rays. Everyone has felt the warmth of the sun’s radiation and intense heat from a charcoal grill or the glowing coals in a fireplace. Most of the heat from these bodies reaches you not by conduction or convection in the intervening air but by radiation. This heat transfer would occur even if there were nothing but vacuum between you and the source of heat.
Black Body Radiation
(i). Emissive Power
The amount of heat energy rediated per unit area of the surface of a body, per unit time and per unit wavelength range is constant which is called as the ’emissive power’ (eλ) of the given surface, given temperature and wavelength. Its S.I. unit is Js-1 m--2.
(ii). Absorptive Power
The ‘absorptive power’ of a surface at a given temperature and for a given wavelength is the ratio of the heat energy absorbed by a surface to the total energy incident on it at a certain time. It is represented by (aλ). It has no unit as it is a ratio.
(iii) Perfect Black Body
A body is said to be a perfect black body, if its absorptivity is 1. It neither reflects nor transmits but absorbs all the thermal radiations incident on it irrespetive of their wavelengths.
(iv) Wein’s Displacement Law
This law states that as the temperature increases, the maximum value of the radiant energy emitted by the black body, move towards shorter wavelengths. Wein found that “The product of the peak wavelength (λm) and the Kelvin temperature (T) of the black body should remain constant.”
`λ_{m}\times T= b`
Where b is constant known as Wein’s constant. Its value is 2.898 x 10-3 mk.
(v) Stefan’s Law
This law states that the thermal radiations energy emitted per second from the surface of a black body is directly proportional to its surface area A and to the fourth power of its absolute temperature T.
Emission coefficient or degree of blackness of a body is represented by a dimensionless quantity ε, 0 < ε < 1. If ε = 1 then the body is perfectly black body. Hence
`E\proptoAT^4`
`E=\sigma AT^4`
Where σ is a Stefan's constant and its value is 5.67 x 10-8 W m-2 K-4.
Newton’s Law of Cooling
According to Newton’s law of cooling, "The rate of loss of heat of a body is directly proportional to the excess of the temperature (T–T0) of the body with respect to the surroundings".
`\frac{-dT}{dt}\propto(T-T_{0})`
Summary
Temperature : The relative measure of hotness or coldness of a body is called its temperature.
Heat : The energy that flows between two bodies by virtue of temperature difference between them is called heat. It flows from a hot body to cold body.
Specific Heat Capacity : The amount of heat per unit mass absorbed or rejected by a substance to change its temperature by one unit is called its specific heat capacity (C).
Molar Specific Heat Capacity : The amount of heat per unit mole absorbed or rejected by a substance to change its temperature by one unit is called its molar specific heat capacity (C).
Calorimeter : A device in which heat measurement can be made is called a calorimeter.
Melting Point : The temperature at which the solid and the liquid states of a substance exist in thermal equilibrium with each other is called its melting point.
Boiling Point : The temperature at which the liquid and the vapour states of the substance coexist is called its boiling point.
Triple Point : The temperature and pressure at which all the three phases of a substance co exist is called its triple point.
Latent Heat : The heat per unit mass required (absorbed or evolved) to change the state of a substance at the same temperature and pressure is called its latent heat.
The temperature of an object is measured with a device called thermometer.
Heat transfer can take place by three modes namely, conduction, convection and radiation. Radiation is fastest of them all and does not require a material medium.