Nuclei Class 12 notes Physics Chapter 13
Introduction
The nucleus contains an entire positive charge and more than 99.9% of the mass of the atom. Rutherford demonstrated from his experiments that the radius of a nucleus is smaller than the radius of an atom by a factor of about 104 and the atomic nucleus is the central core of every atom.
In this chapter, we shall study the constituents of the nucleus and how they are held together. We shall discuss various properties of nuclei such as size, mass, density, and stability of nuclei and associated nuclear phenomena such as radioactivity, nuclear fission, and nuclear fusion.
Atomic Mass Unit
The unit in which atomic and nuclear masses are measured is called atomic mass unit (u), defined as `\frac{1}{12}th` of the mass of an atom of 6C12 isotope.
`1u=\frac{1}{12}\times\frac{12}{6.02\times 10^{23}}`
`1u=1.66\times 10^{-27}kg`
1amu = 931 MeV
Composition of Nucleus
(i). Atomic Number
It is the number of protons present inside the nucleus of an atom of the element. It is represented by Z.
(ii). Mass Number
It is the total number of protons and neutrons present inside the atomic nucleus of the element. It is represented by A.
A = Z + N
Nuclear species or nuclides are represented as ZXA where X is the chemical symbol of the species.
Recommended Books
- NCERT Textbook For Class 12 Physics Part 1 & 2
- CBSE All In One Physics Class 12 2022-23 Edition
- Oswaal CBSE Chapterwise Question Bank Class 12 Physics Book
- Modern's abc Plus of Physics for Class-12 (Part I & II)
Read also: Semiconductor Electronics: Materials, Devices and Simple Circuits Chapter 14 Physics Notes
(iii). Isotopes
All nuclides with the same atomic number but a different mass number are called isotopes. Ex- 1H1 (Protium), 1H2 (Dutirium) and 1H3 (Tritium) are isotopes.
(iv). Isobars
Nuclides with the same mass number A but a different atomic number are called isobars. Ex- 1H3 and 2He3 are isobars.
(v). Isotones
Nuclides with the same neutron number N but different atomic numbers Z are called isotones. Ex- 6C14, 7N15 and 8O16 are isotones.
(vi). Discovery of Neutron
Neutron was discovered experimentally by Chadwick in the year 1932 and was awarded Nobel Prize in Physics in 1935 for their discovery. A neutron is a neutral particle carrying no charge and having a mass roughly equal to the mass of a proton.
Now the mass of a neutron is known to have a high degree of accuracy and is equal to mn = 1.67 x 10-27 kg. A free neutron is unstable and has a mean life of 1000 seconds. Whereas a free proton is stable. The neutron is however stable inside the nucleus.
Read also: Amines Chemistry Class 12 Notes Chapter 13
Size of the Nucleus
It has been found by performing scattering experiments in which fast electrons, instead of α-particles (projectiles) that bombard targets made up of various elements a nucleus of mass number A has a radius.
R = R0A1/3
where R0 = 1.2 × 10–15 m.
Mass-Energy and Nuclear Binding Energy
(i). Mass-Energy
Einstein gave the famous mass-energy equivalence E = mc2, here the energy equivalent of mass m is related by the above equation and c is the velocity of light in a vacuum and is approximately equal to 3 × 108 m/s. Einstein's mass-energy relation has been experimentally verified in the study of nuclear reactions amongst nucleons, nuclei, electrons, and other more recently discovered particles.
(ii). Nuclear Binding Energy
The nucleus is made up of neutrons and protons. Therefore the mass of the nucleus (M) should be equal to the total mass of its protons and neutrons. However, it is found to be always less than this. This difference in mass (ΔM) is called the mass defect and is given by
ΔM = [Zmp + (A – Z)mn] – M
It is a mass defect that appears in the form of binding energy, responsible for binding the nucleons together in the nucleus.
Nuclear Force
To bind a nucleus together there must be strong nuclear forces of attraction that hold together the nucleons (neutrons and protons) in the tiny nucleus of an atom, to overcome the repulsion between the (positively charged) protons. This attractive force is called nuclear force.
Read also: Conceptual Questions for Class 12 Physics Chapter 13 Nuclei
Radioactivity
The process of spontaneous disintegration shown by some unstable atomic nuclei is known as natural radioactivity. This property is associated with the emission of certain types of penetrating radiations, called radioactive rays, or Becquerel rays (α, β, γ-rays).
 |
| Radioactive Radiation |
The elements or compounds, whose nuclei disintegrate and emit radiation are called radioactive elements. Radioactivity is a continuous, irreversible nuclear phenomenon.
Radioactive Decays
Generally, there are three types of radioactive decays
`U_{92}^{238}\rightarrow He_2^4+Th_{90}^{234}`
(i). α decay
In this process, the parent nucleus disintegrates to give a daughter nucleus and helium nucleus or an alpha-particle. The mass number of the daughter nucleus decreases by four units and the atomic number decreases by two units. A typical example of this decay mode is
`U_{92}^{238}\rightarrow He_2^4+Th_{90}^{234}`
Thus, the daughter nucleus is shifted in the periodic table by 2 units in the backward direction.
Properties of Alpha Particle
The alpha particles are helium nuclei with 2 units of positive charge and a mass of 4 amu.
Because they are charged particles so they can be deflected by electric and magnetic fields.
They ionize the medium through which they pass. The ionizing power of Alpha-particles is much high, about 100 times that of Beta-particles and 10000 times that of Gamma-rays.
Alpha particles are scattered while passing through very thin metal foils.
The penetrating power of Alpha-particles is very small as compared to that of Beta-particles and Gamma-rays.
Long exposure to alpha emitters produces harmful effects on the human body.
(ii). β decay
In this process, the parent nucleus disintegrates to give a daughter nucleus and a Beta-particles. Beta-decay is classified into three categories.
a) Electron emission or β--decay
Here, the parent nucleus disintegrates to give a daughter nucleus, a Beta-particles and a new particle called an antineutrino. The mass number of the daughter nucleus remains the same and the atomic number increases by one unit.
`C_6^{12}\rightarrow N_7^{14}+\beta^{-}+\overline\nu`
b) Positron Emission or β+-decay
In this process, the parent nucleus disintegrates to give a daughter nucleus, a Beta-particles, and a new particle called the neutrino. The mass number of the daughter nucleus remains the same and the atomic number increases by one unit.
`Na_{11}^{22}\rightarrow Ne_{10}^{22}+\beta^++\nu`
c) Electron Capture
Here the parent nucleus captures one of the orbital electrons with the emission of a neutrino. The mass number of the daughter nucleus remains the same and the atomic number increases by one unit.
`Mn_{25}^{54}+\beta^{-}\rightarrow Cr_{24}^{54}+\nu`
Thus, daughter nucleus is shifted in periodic table by 2 unit in forward direction.
Properties of Beta-particles
Beta-particles are negatively charged particles whose rest mass and electric charges are exactly the same as that of electrons. So, the rest mass of a Beta-particle is 9.1×10-31 kg and its negative charge is e = 1.6×10-19 C.
The penetrating power of Beta-particles is comparatively large. Their penetrating power is about 100 times that of Alpha-particles but about 1% of that of Gamma-rays.
Beta-particles ionize the medium through which they pass. However, their ionizing power is very small as compared to that of Alpha-particles.
Beta particles may be dangerous and any contact with the body should be avoided.
Because they are charged particles so they can be deflected by electric and magnetic fields.
(iii). γ decay
Alpha and beta decay of a radioactive nucleus leave the daughter nucleus in an excited state. If the excitation energy available with the daughter nucleus is not sufficient for further particle emission, it loses its energy by emitting electromagnetic radiations, also known as Gamma-rays. Mass and charge of the daughter nucleus remain the same as before the emission of Gamma-rays.
`Ba_{56}^{137}\rightarrow Ba_{56}^{137}+\gamma`
Properties of Gamma-rays
Gamma-rays are electromagnetic waves of extremely short wavelengths. So, these are not deflected either in an electric field or in a magnetic field.
Gamma-rays travel at the speed of light.
Gamma-rays carry a large amount of energy.
The penetrating power of Gamma-rays is extremely large. The penetrating power of Gamma-rays is about 100 times that of Beta-particles and 10000 times of Alpha-particles.
Gamma-rays too ionize the medium through which they pass. However, their ionizing power is about 1% that of Beta-particles and only about 0.01% as compared to that of Alpha-particles.
Law of Radioactive Decay
According to the law of radioactive disintegration, the rate of spontaneous disintegration of a radioactive element is proportional to the number of nuclei present at that time.
Mathematically, it can be written as
`\frac{dN}{dt}\infty N................(1)`
where N is the number of atoms present at time t. By removing the proportionality sign, we get
`\frac{dN}{dt}=-\lambda N............(2)`
where λ is a constant of proportionality and is known as the decay constant of the element. The negative sign indicates that as t increases N decreases.
`\frac{dN}N=-\lambda dt.............(3)`
Integrating both sides, we have
`\int\frac{dN}N=-\lambda\int dt`
`\log_e(N)=-\lambda t+C.............(4)`
where C is the constant of integration and is evaluated by the fact that at t = 0, the number of atoms of the radioactive element is N0. Using this condition, we get
`C=\log_e(N_0).................(5)`
Substituting this value of C in Eq. (5), we get
`\log_e(N)=-\lambda t+\log_e(N_0)`
`\log_e(N)-\log_e(N_0)=-\lambda t`
Thus,
`N=N_0e^{-\lambda t}.................(6)`
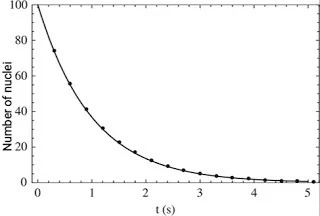 |
| Exponential decay curve |
Units of Activity
The SI unit of activity is named after Becquerel. The activity of a radioactive sample is said to be 1 becquerel if the rate of decay is 1 nucleus per second.
Therefore,
becquerel = 1 Bq = 1 decay/sec
An older unit Curie (Ci) is still commonly used to measure activity.
Therefore,
1 curie = 1 Ci = 3.7 × `10^{10}` decay/sec = 3.7 × `10^{10}` Bq
Yet, another unit of activity of radioactive sample is rutherford (Rd) and is defined as :
1 rutherford = 1 Rd = `10^{10}` decay/sec = `10^{10}` Bq
Half-life
Half-life is the time at which the activity of sample has been reduced to one-half of its initial value.
If N0 is the initial number of radioactive nuclei present, then in one half-life this number will reduce to N0/2. Therefore,
`N=\frac{N_0}2`
substituting N in Eq. (6), we get
`\frac{N_0}2=N_0e^{-\lambda t_{1/2}}`
`\1/2=e^{-\lambda t_{1/2}}`
`e^{\lambda t_{1/2}}=2`
Taking natural log on both sides,
`\lambda t_{1/2}=\log_e2`
`t_{1/2}=\frac{2.303\log_{10}\2}\lambda`
`[ \log_ex=2.303\log_{10}x\ ]`
`t_{1/2}=\frac{0.693}\lambda`
Therefore, the half-life of a radioactive material depends upon the disintegration constant. The larger the value of the disintegration constant, the smaller the half-life. The half-life of radioactive material is the intrinsic property it cannot be altered by any physical or chemical means.
Soddy and Fajan’s Displacement Laws
(i) α-decay: The emission of one α-particle reduces the mass number by 4 units and atomic number by 2 units. If parent and daughter nuclei are represented by symbols X and Y respectively then,
`X_{Z}^{A}\rightarrow Y_{Z-2}^{A-4}+He_{2}^{4}`
(ii) β-decay: In β-decay, the daughter nucleus has an atomic number greater than one but the same mass number as that of the parent nucleus. When a nucleus emits a beta particle, one of its neutrons breaks into a proton, an electron (i.e., β-particle), and an antineutrino.
`X_{Z}^{A}\rightarrow Y_{Z+1}^{A}+β+\vec{\nu}`
(iii) γ-decay: When parent atoms emit gamma rays, no charge is involved as these are neutral rays. Thus there is no effect on the atomic number and mass number of the parent nucleus. The emission of these rays changes the nucleus from an excited state to a less excited state.
Note: In a magnetic field, γ-rays are undeviated and β-particles are the most deviated.
Nuclear Energy
(i). Nuclear Fission
The process of the splitting of a heavy nucleus into two or lighter nuclei is called nuclear fission. When a slow-moving neutron strikes a uranium nucleus (92U235), it splits into 56Ba144 and 36Kr89 along with three neutrons and a lot of energy.
`U_{92}^{235}+n_{0}^{1}\rightarrow Ba_{56}^{144}+Kr_{36}^{89}+3n_{0}^{1}`
(ii). Nuclear fusion
The process of combining two lighter nuclei to form one heavy nucleus is called nuclear fusion.
`H_{1}^{2}+H_{1}^{2}\rightarrow He_{2}^{3}+n_{0}^{1}`
In this process, a large amount of energy is released. The hydrogen bomb is based on nuclear fusion. The source of the Sun’s energy is the nuclear fusion taking place at the sun.
Summary
Atomic number: The number of electrons outside the nucleus or protons in a nucleus is known as the atomic number. It is represented as Z.
Mass number: The sum of the number of protons (Z) and the number of neutrons (N) is called the mass number. It is denoted by A.
Nucleons: Protons and neutrons in a nucleus are collectively known as nucleons.
Isotopes: Nuclides have the same atomic number but the different mass numbers of a particular element are called isotopes.
Isobars: Nuclides having the same mass number but different atomic numbers of different elements are called isobars.
Isotones: Nuclides having the same number of neutrons but different atomic numbers are called isotones.
Mass defect: Difference between the mass of the constituent nucleons of the nucleus in the free state and the mass of the nucleus.
Binding energy: The total energy required to disintegrate the nucleus into its constituents particles (i.e., nucleons) is called the binding energy of the nucleus.
Radioactivity: The phenomenon of spontaneous emission of radiation by heavy elements is called radioactivity.
Law of radioactive decay: The rate of disintegration of a radioactive substance at an instant is directly proportional to the number of nuclei in the radioactive substance at that time.
Half-life: The time during which half of the atoms of the radioactive substance disintegrates is called the half-life.
Mean life: It is given by the sum of the total lifetime of all atoms divided by the total number of atoms present.
Nuclear fission: Nuclear fission is the process of splitting a heavy nucleus into two lighter nuclei along with the conversion of the mass defect into energy.
Nuclear fusion: Nuclear fusion is a process in which two very light nuclei combine to form a nucleus with a large mass number along with releasing a large amount of energy is called nuclear fusion.
On the atomic scale, mass is measured in the atomic mass unit (u). 1 atomic mass unit (1 u) is `\frac{1}{12}th` mass of one atom of 12C.
Radius of nuclei R = R0A1/3, R0 = 1.2 fm.
Neutrons and protons are bound in a nucleus by the short-range strong nuclear force. The nuclear force does not distinguish between neutron and proton.
The difference in mass of a nucleus and its constituents is called the mass defect ΔM = [Zmp + (A – Z) mn] – M
The binding energy of the nucleus Eb = ΔMc2.
Radioactivity is the phenomenon in which nuclei of a given species transform by giving out α-rays (helium nuclei), β-rays (electrons), and γ-rays.
The half-life (T1/2) of a radionuclide in the time in which N has been reduced to one-half of its initial value.
The mean life (τ) is the time at which N has been reduced to e-1 of its initial value.
Energy is released when less tightly bound nuclei are changed into more tightly bound nuclei.
In fusion, lighter nuclei combine to form a larger nucleus. The fusion of hydrogen nuclei into helium nuclei is the source of energy for all-stars including our sun.

